
Promising drug-development systems get new U.S. funding
Kidney disease researchers at the University of Washington will use a multimillion-dollar federal grant to advance drug-development platforms that would reduce reliance on animal tests in the production of future medications.
The investigators, in the UW schools of Medicine and Pharmacy, received the $7.3 million award from the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health.
Their research aims to finalize multiple kidney-tissue platforms for drug testing, and submit those platforms to the U.S. Food and Drug Administration for approval.
“We are incredibly pleased to receive this award. It’s an exciting project that will be the capstone for our already 10-plus years of NCATS funding for kidney-on-a-chip and kidney organoid research,” said Dr. Jonathan Himmelfarb, a professor of nephrology at the UW School of Medicine.
Since the 1960s, few new medications have emerged for people with chronic kidney diseases. Drug development is slow and expensive and relies heavily on animal testing to confirm the effectiveness and safety of candidate formulations.
According to the U.S. Congressional Budget Office, only about 12% of the drugs that enter the FDA’s clinical trials process eventually reach patients.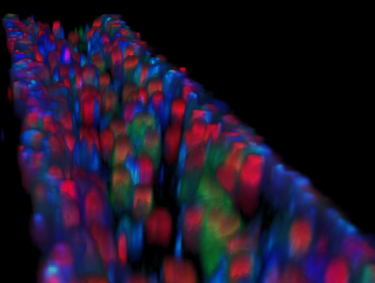
These tissue chips are engineered platforms that incorporate three-dimensional architecture and fluid flow, making them superior to other systems in mimicking human kidney function.
Developed over the past decade, these systems use human kidney cells, including stem cells, to enable scientists to create human models of disease, and then test novel drugs and define new therapeutic strategies.
The funded project is formally known as the UW Translational Center for Microphysiological Systems. Himmelfarb will direct the effort. The platforms’ development will be led by Benjamin Freedman, associate professor of nephrology in the School of Medicine, and Ed Kelly, associate professor of pharmaceutics in the School of Pharmacy. The platforms’ qualification process will be driven by Catherine Yeung, associate professor of pharmacy, and Ying Zheng, associate professor of bioengineering.
“The FDA Modernization Act 2.0 permits the use of alternative approaches to animal testing for predicting drug safety prior to initiation of trials in humans,” said Kelly. “As a toxicologist, being able to use alternative technologies to animal testing is an important advancement, with respect to the 3Rs of toxicology: reduce, refine and replace animal testing.”
Alternatives named in the Modernization Act include microphysiological systems such as organoids and organs-on-chips, he added. “Our group has developed kidney models for both alternatives and with this funding we propose to qualify the models to meet FDA regulatory requirements.”