


Novel screening test effective for complications from liver disease
Drs. Philip Vutien, assistant professor and George Ioannou, professor (Gastroenterology) and colleagues found that a novel and portable screening system using a smartphone app is a more accessible option for individuals to test their own risk of hepatic encephalopathy, a complication of chronic liver disease.
Liver disease and hepatic encephalopathy
Learn More
Hepatic encephalopathy is an often-temporary neurological disorder that arises when the liver is unable to remove toxins from the bloodstream. Toxins build up and travel to the brain affecting brain function and causing cognitive impairment.
4.5 million adults in the U.S. have chronic liver disease (CLD). Liver disease is caused by genetic, infectious or external factors and can inhibit the liver from functioning properly. CLD is a continuous process of inflammation, destruction, and regeneration of liver tissue, which leads to fibrosis and cirrhosis. Left untreated, the damage can be irreversible.
Multiple bodily functions can be impacted by chronic liver disease, including neurological disorders such as minimal hepatic encephalopathy. Up to 50% of people with cirrhosis of the liver eventually develop symptoms of hepatic encephalopathy.
Screening for minimal hepatic encephalopathy is important for patients with CLD as the symptoms can worsen (overt hepatic encephalopathy) causing serious complications including loss of consciousness or coma. Timely diagnosis and treatment can help to alleviate symptoms as well as to minimize progression of liver damage.
critical flicker frequency screening
One of the screening options to test for hepatic encephalopathy is conducted using critical flicker frequency (CFF), a well-studied neurophysiologic screening test that measures responsiveness to a flickering light source as it changes frequency over time.
Currently available CFF devices, such as the Flicker Fusion System (Lafayette-FFS), are large, expensive, and designed for use in professional medical settings. To address these limitations, UW and UMass Amherst scientists developed Beacon, a novel, portable device that measures CFF and is administered by a smartphone app.
screening using a smartphone
The Beacon system consists of 2 components: the light stimulus source with wireless controller and battery base and an application running on a smartphone (connected to the light source via Bluetooth) for user input and to record results. The application opens with instructions for taking the test and, once started, the user indicates by tapping the screen when they begin to see the light flicker. At the end of the test, they receive their CFF measurement in Hz – an indication of at which frequency they were able to perceive the flicker of the light.
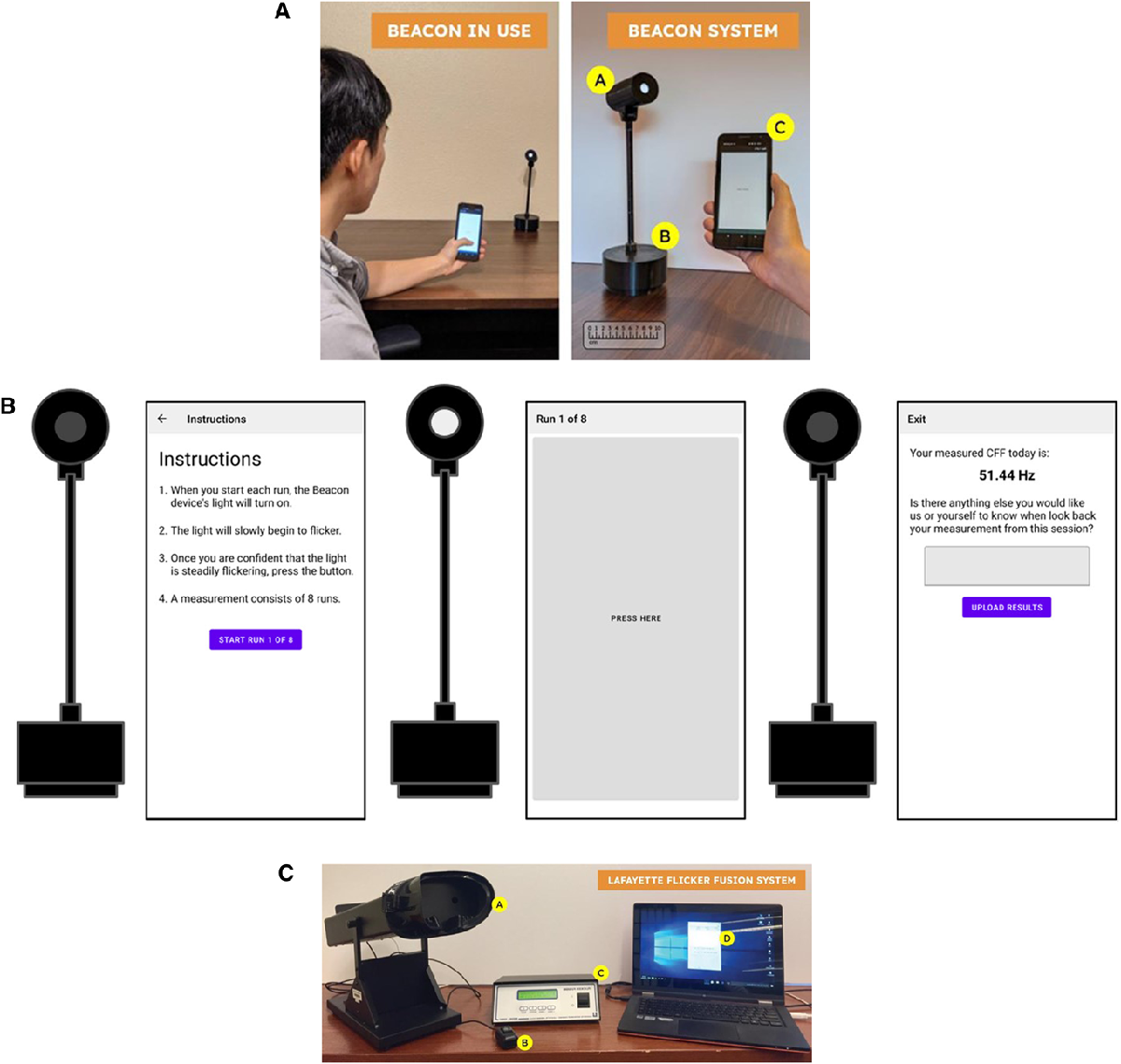
Efficacy of the Beacon system was tested among study participants from the hepatology clinics of the University of Washington and compared to participants who tested using the Lafayette-FFS. Overall, Beacon-derived CFF readings were reliable and demonstrated good correlation with those obtained using the commercially available Lafayette-FFS device.
With a cheaper and self-administrable (though no less accurate) screening option available, testing for hepatic encephalopathy can be much more accessible.
Learn more
This study was published in the American Journal of Gastroenterology by Drs. Philip Vutien, assistant professor and George Ioannou, professor (Gastroenterology) along with colleagues from UW Computer Science, UW Human Centered Design and Engineering and UMass Amherst Information and Computer Science.